Ice forms when the temperature is below freezing 0Celsius or 32Fahrenheit. Pressure Effects Changes in presure have very little effect on the volume of a liquid.
As temperatures rise solids turn into liquids and liquids turn into gases As temperatures rise solids turn into liquids and liquids turn into gases.

. Climate change intensifies this cycle because as air temperatures increase more water evaporates into the air. If you get water hot enough the molecules move so much that the hydrogen bonds that hold. In contrast the water may be warm during the summer.
As the temperature of a solid liquid or gas increases the particles move more rapidly. Ice gain heat in the interval of points A and B and its temperature becomes 0 ºC that is the melting point of it. Yes the pH is inversely proportional to the temperature of the solution.
As a liquid the attractive forces between molecules weaken and individual molecules can begin to move around each other. When we consider this property of water having less density when in solid state ice than liquid water which has a maximum density at a temperature of 4 0 c we see that ice tends to float on water. The constancy of temperature when water is going through its phase changes at 0C and 100C provides some insight into the nature of the internal energy of water ice and steam and into the nature of temperature.
Gained heat is spent on t breaking the bonds of molecules. The large size of the phase change energy indicates that there is a large amount of potential energy associated with the intermolecular forces between the water. Temperature plays a significant role on pH measurements.
Warmer air can hold more water vapor which can lead to more intense rainstorms causing major problems like extreme flooding in. The dissociation of water into hydrogen and hydroxide ion can be. Excessive rainfall due to the water cycle can cause floods.
If a liquid is heated sufficiently it forms a gas. Owing to temperature changes the pH value of the solution changes. Water temperature affects viscosity which in turn affects ionic activity and conductivity.
Ionic mobility is dependent on viscosity which is in turn dependent on temperature 13. The phase velocity of a gravitycapillary wave on a fluid interface is influenced by the effects of gravity surface tension density and by fluid inertia. Deficient rainfall lack of adequate water sources can lead to draught in a given region.
How does the temperature affect the phase of water. When the temperature of the water becomes 100 ºC it starts to boil and evaporation of it speeds up. When water is cooled to form ice the hydrogen bonds become more rigid but also more open causing water to expand thus increasing in volume.
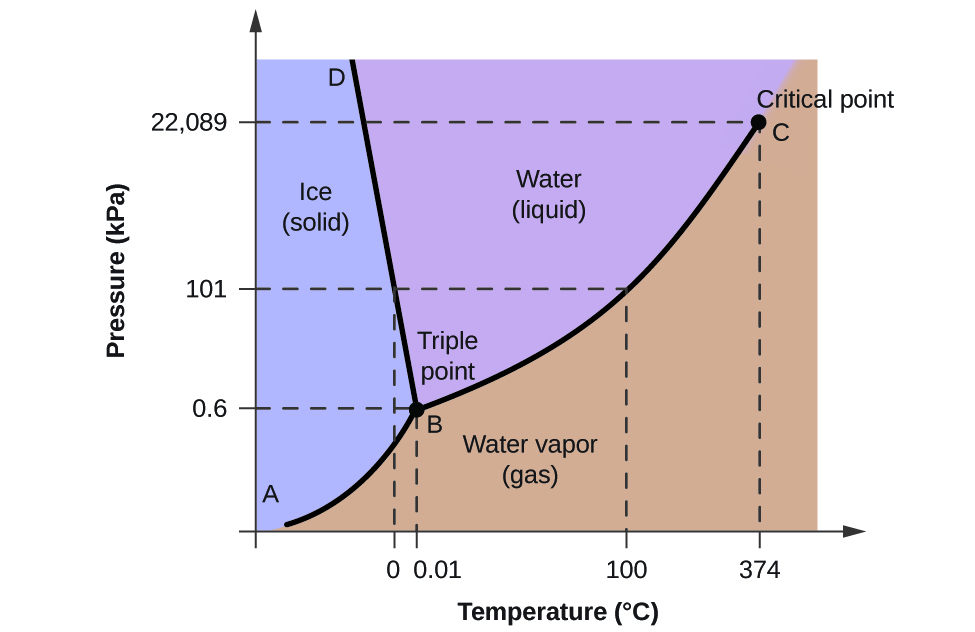
When the temperature of a solution rises the molecular vibrations in the solution rise resulting in the ionization and formation of H ions. For water the critical pressure is very. We have only ice in the 1 st region.
The hydrogen bond gives water this unique property. Sources of water - waterways fed by snowmelt will be cold during the spring and summer while waterways fed by hot springs may keep water warm throughout the year. At the beginning we have ice at -20 ºC.
During the winter water may freeze. Climate change affects evaporation and precipitation. The critical pressure P c is the pressure that must be applied to the gas at the critical temperature in order to turn it into a liquid.
These are some of the basic weather conditions that are directly influenced by the water cycle. More evaporation is causing more precipitation on average. Temperature-10 C 10 C 50 C 90 C 110 C 120 C Predicted phase solid liquid or gas Actual phase solid liquid or gas 4.
Phase Transition of Water. After melting process completed in the 3rd region there is only water and temperature of water starts to increase. If a liquid is cooled sufficiently it forms a solid.
At any temperature higher than that the gas phase cannot be made to liquefy no matter how much pressure is applied to the gas. Temperature causes water molecules to move more quickly because each individual molecule has more energy as it gets hotter according to Kinetic molecular theory. This graph shows the phase diagram of water.
Temperature affects phase change by slowing down the movement in between the atoms thus causing a change in kinetic energy which in turn causes the atoms to undergo forms of combining or a type of disepersion. Molasses and mercury are more viscous than water. Put simply water evaporates from the land and sea which eventually returns to Earth as rain and snow.
There are three factors that affect the rate at which a solid dissolves in water. A study shows that daily variations of water temperatures are generally small for cold headwater streams but increase as streams get larger in size because bigger streams become less dominated by groundwater and are more exposed to meteorological conditions the mean daily water temperature increases in a downstream direction and water temperature is generally. As the temperature rises molecular vibrations increase which results in the ability of water to ionise and form more hydrogen ions.
Rainfall and snow are results of the water cycle and can lead to severe weather conditions. As a result the pH will drop. Water like most things in nature expands when heated and contracts when cooled except in the temperature range between 4 o Cs and 0 o C.
The three factors that affect the rate are stirring temperature. Since surface tension and density are affected by temperature the phase velocity begins to become slightly affected by changes in temperature. The more viscous it is the less fluid it is.
The geometry leads to a rigid rather open hexagonal structure. 2nd region includes both water and ice. At 37399C particles of water in the gas phase are moving very very rapidly.
Climate change is likely causing parts of the water cycle to speed up as warming global temperatures increase the rate of evaporation worldwide. Viscosity refers to a liquids ability to resist flow 23. When ice is warmed above freezing it melts and becomes liquid water.
As the temperature falls the particles slow down. How does temperature affect the movment of water molecules. While testing your predictions you may have noticed that there were specific temperatures at which the phase of the water always changed.
Seasons - air temperature affects water temperature. Kinetic energy while being the reason phase changes are constant Kinetic Energy can be caused by other means. As you can see between the points B and C temperature of the mass does not change because its state is changing in this.
More H ions lead to more acidic behavior. In ice a water molecule has four nearest neighbors to which it is bonded via hydrogen bonds.

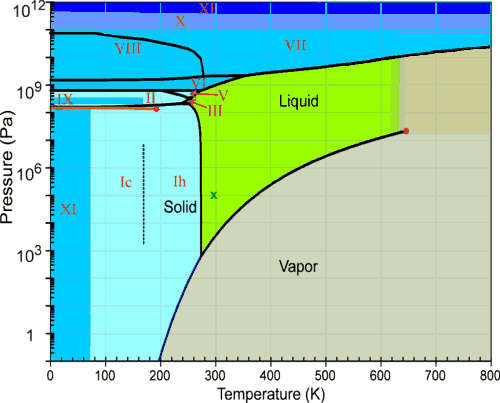
Phase Diagram For Water Chemistry For Non Majors

0 Comments